Featured China G Products
-
-
GF109203X 133052-90-1
;2-(1-(3-Dimethylaminopropyl)-indol-3-yl)-3-(indol-3-yl)maleimide;BisindolylmaleimideGF 109203X;3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1H-indol-3-yl)-1H-pyrrol... >>>
 Toda Biotechnology Co., Ltd
Toda Biotechnology Co., Ltd

-
GGH packing seal shell and tube graphite heat exchanger
... >>>
 Nantong Xingrui Heat-exchanging Containers Co., Ltd. (Nantong Xingqiu Graphite Equipment Co.,Ltd)
Nantong Xingrui Heat-exchanging Containers Co., Ltd. (Nantong Xingqiu Graphite Equipment Co.,Ltd)

-
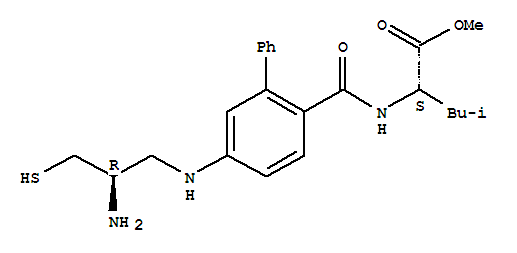
GGTI-286 171744-11-9
L-Leucine,N-[[5-[(2-amino-3-mercaptopropyl)amino][1,1'-biphenyl]-2-yl]carbonyl]-, methylester, (R)-; GGTI 286... >>>
 Toda Biotechnology Co., Ltd
Toda Biotechnology Co., Ltd
-
GF109203X 133052-90-1
Total 3238 Products,Showing 97 / 324|‹ ‹‹ 91 92 93 94 95 96 97 98 99 100 ›› ›|










