| Product description : |
Quick Details:
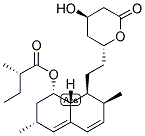
Name: Lovastatin
CAS NO: 75330-75-5
MF: C24H36O5
MW: 404.55
Appearance: white powder
Standard:USP
Package:25kg/drum
Storage: shading, sealed.
Use: it is mainly used for the treatment of hypercholesterolemia and mixed hyperlipidemia
Description:
Lovastatin (Merck's Mevacor) is a statin drug, used for lowering cholesterol (hypolipidemic agent) in those with hypercholesterolemia to reduce risk of cardiovascular disease. Lovastatin is a naturally occurring compound found in food such as oyster mushrooms, red yeast rice,and Pu-erh.
Specification:
Test Items Standard Result
Description A white to off-white, crystalline powder Conforms
Identification IR/UV Conforms
Specific optical rotation +324°- +338° +326.8°
Loss on drying ≤0.3% 0.10%
Sulphated ash ≤0.2% 0.03%
Heavy metals ≤20ppm <20ppm
Related compound A ≤0.5% 0.24%
Assay(HPLC) 98.5%-101.0% 99.5%
HPLC
Purity RRT0.66 ≤0.15% 0.06%
RRT0.92 ≤0.15% 0.09%
RRT1.49 ≤0.15% 0.14%
RRT2.43 ≤0.15% 0.15%
Max unknown impurity ≤0.10% 0.08%
Total impurity ≤1.0% 0.66%
Residual solvents Ethanol ≤5000ppm 410ppm
Butyl Acetate ≤5000ppm 120ppm
Conclusion The results conform with USP35 standard
Patent Disclaimer:
1. Products protected by valid patents are not offered for sale in jurisdictions where the sale of such products constitutes a patent infringement. The current list only reflects the products and technologies that are available, under development: note that some products may be developed or produced for internal and experimental uses with no commercal aim.
2. Sales of products are limited to those allowed by Chapter VII PLPRC 63, the above includes Research and development quantities.
3. R&D use in accordance with (1) 35 USC 271(e)+A13(1) in the U.S.; (2) Section 69.1 of Japanese Patent Law in Japan; (3) Section 11, No. 2 of the German Patent Act of 1981 in Germany; (iv) Section 60, Paragraph 5b of the U.K. Patents Act of 1977 in the U.K.; (4) Sections 55.2(1) and 55.2(6) and other common law exemptions of Canadian patent law; (5) Section 68B of the Patents Act of 1953 in New Zealand together with the amendment of same by the Statutes Amendment Bill of 2002; (6) such related legislation and/or case law as may be or become applicable in the aforementioned countries; and (7) such similar laws and rules as may apply in various other countries.
Competitive advantages:
Well and High Quality Control ,
Prompt Delivery ,
Competitive Prices ,
Earth-friendly Products ,
Small Order Acceptable
|
| Synonyms : |
mevlor;msd803;oxy)-1-naphthaleneheptanoicaciddelta-lactone
;Lovastation;Lovastin;Lovalip;Mevinolin;8-[2-(4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2-methylbutanoate;(1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate;(1S,3R,7S,8S)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate;Red Yeast Rice P.E; |